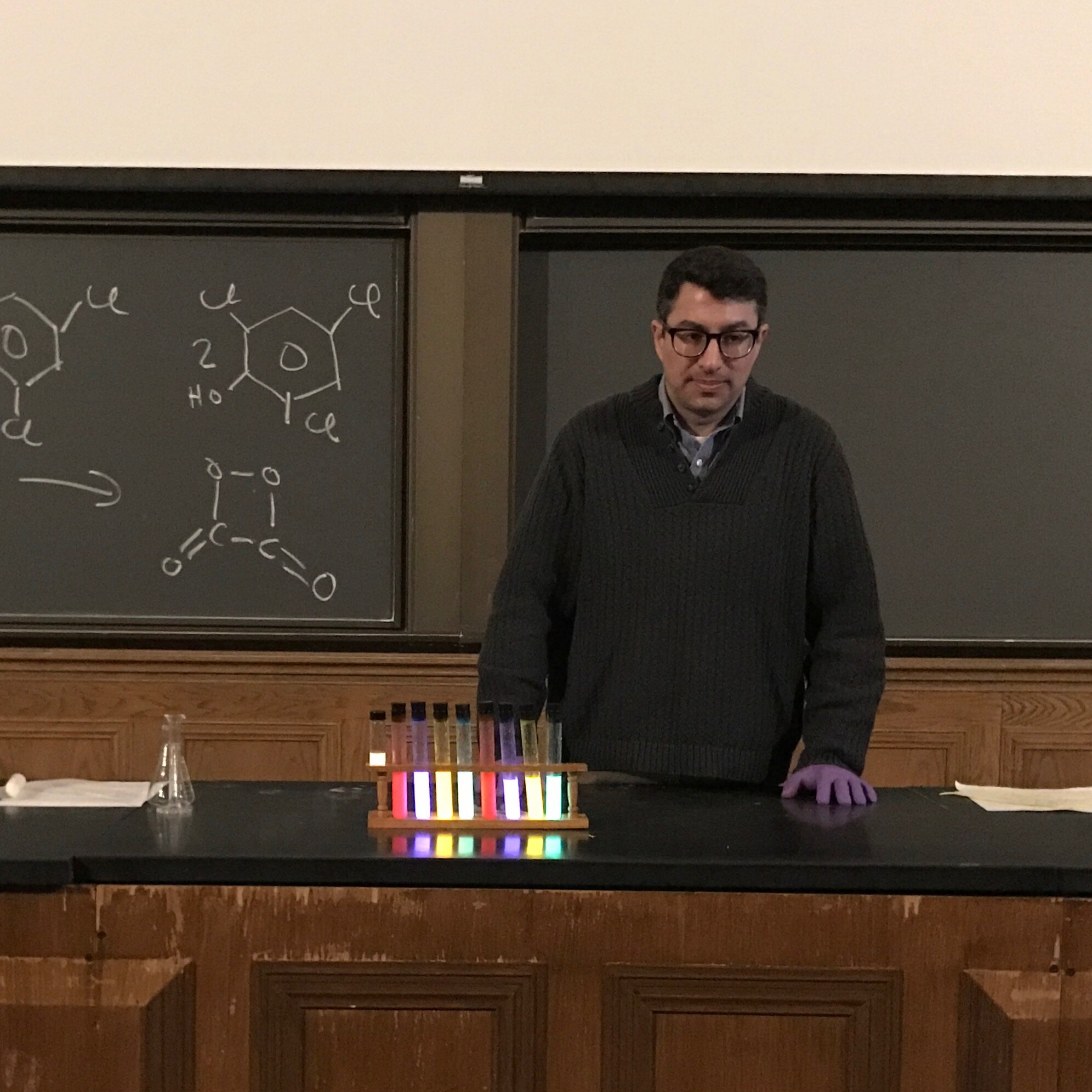
Chemistry Menorah
Inspired by a YouTube video on how to make your own glowstick reaction, we enlisted the help of Professor Aaron Dinner of the University of Chicago Chemistry Department to create a glowing chemistry menorah. Instead of a shamash (helper candle), we used a beaker of hydrogen peroxide, which initiated the chemical reaction that caused the test tubes to glow. While we encourage you to consider your own interpretations, we found it thought provoking to imagine that people looking at a chemical reaction like this 2,000 years ago would have considered it a miracle!
The chemicals we used:
10 mL diethyl phthalate (solvent)
100 mg sodium acetate (to set the pH)
50 mg TCPO
3 mL 30% hydrogen peroxide
3 mg of fluorescent dye
The dies we used:
9,10-bis(phenylethynyl)anthracene for green
rubrene for yellow
9,10-diphehylanthracene for blue
rhodamine B for red
We got the test tubes and rack from Amazon:
Special thanks to the University of Chicago Chemistry Department, and especially Dr. Meishan Zhao, for helping us obtain the chemicals, and thanks also to Hannah Dinner, Solomon Dinner, and Miriam Niestat for their assistance and patience in filming the experiment.
(If you like this idea but don't want to set up all the chemistry, check out our menorah ideas using regular glowsticks, which can be purchased at Amazon or elsewhere.)